Analytical Method Development
When is Analytical Method Development required ?
Analytical Method Development is required for :
» Herbal Products
» New process and reactions
» New molecules
» Active ingredients (Macro analysis)
» Residues (Microanalysis)
» Impurity Profiling
» Component of Interest in different matrices
What does analytical methodology consist of ?
An Analytical Methodology consists of the following
» Techniques
» Method
» Procedure
» Protocol
What does analytical methodology provide to an analyst ?
» The required data for a given analytical problem
» The required sensitivity
» The required accuracy
» The required range of analysis
» The required precision
i.e. the minimum requirements which essentially are the specifications of the method for the intended purpose: To be able to analyse the desired analyte in different matrices with surety and certainity.
What are the capabilities of SRI in method development ?
SRI has the trained expertise and all the state-of-the-art-facilities required for developing new methods of analysis for all kinds of components in different complex matrices. The methods developed by SRI can be used by other laboratories. The capability of SRI in this area has been acknowledged by the international bodies concerned with the quality standards and systems.
What is Method Validation / Evaluation ?
Method validation is the process of documenting or proving that :
Analytical Method provides analytical data for the intended use
Why is analytical method validation required ?Method Validation is required for the following reasons
» Assuring quality and achieving 3 a levels
» Achieving acceptance of products by the international agencies.
» Mandatory requirement purposes for accreditation as per ISO 17025 guidelines
» Mandatory requirement for registration of any pharmaceutical product or Pesticide formulation
» Validated methods are only acceptable for undertaking proficiency testing.
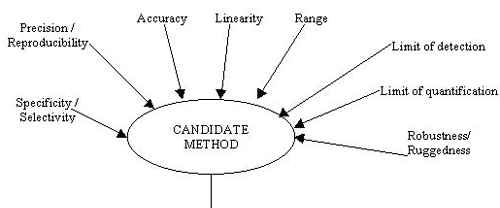
What are the various parameters studied for Method Validation ?
» For validation the developed method is subjected to following studies :
» Precision / Reproducibility
» Accuracy
» Linearity
» Specificity / Selectivity
» Limit of detection
» Limit of quantitation
» Robustness / Ruggedness